Cancer could be detected 3 years before symptoms appear with a simple blood test; new study reveals
A groundbreaking new study from Johns Hopkins University suggests that a simple blood test could detect cancer up to three years before symptoms manifest. This early detection could revolutionize cancer treatment and significantly improve survival rates.
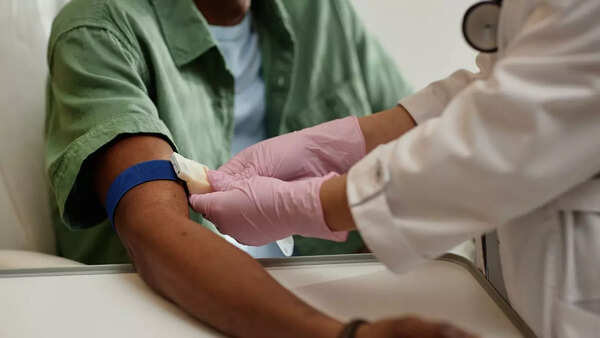
Early Detection: A Game Changer in Cancer Treatment
Cancer remains a leading cause of death worldwide, with late diagnosis being a major obstacle in effective treatment. Detecting cancer in its early stages is critical, as tumors are typically smaller, less aggressive, and more responsive to therapy. According to researcher Yuxuan Wang from Johns Hopkins, detecting cancer "three years earlier provides time for intervention," potentially making the difference between a curable and life-threatening condition.
This advantage is especially crucial for aggressive forms of cancer where early intervention is paramount.
The Science Behind the Blood Test
The research focuses on circulating tumor DNA (ctDNA), which are fragments of DNA that tumors shed into the bloodstream. These fragments are often present in very low concentrations, especially in the early stages of cancer, making them difficult to detect.
To overcome this challenge, scientists developed sophisticated algorithms and cross-checking methods to analyze blood samples for specific DNA patterns associated with cancer. This technique forms the basis of a Multi-Cancer Early Detection (MCED) test, designed to identify cancer-specific genetic changes in the blood.
Study Results: A Promising Outlook
The research team analyzed blood samples from 52 individuals, divided into two groups:
- 26 people who were diagnosed with cancer within six months of sample collection.
- 26 people who remained cancer-free.
The MCED test flagged eight cancer cases, representing a 31% detection rate. This detection occurred before any formal diagnosis or visible symptoms, highlighting the potential of the test for early cancer detection.
Further analysis of older blood samples from participants revealed that cancer signals were present up to 3.5 years before diagnosis in four out of six cases. While the ctDNA levels were lower than the current test threshold, this finding suggests that tumors begin shedding DNA into the blood long before symptoms appear.
Challenges and Future Directions
While the results are encouraging, the study also underscores the need to improve the sensitivity of current technology. Dr. Bert Vogelstein, a senior cancer researcher, emphasizes that the study "shows the promise of MCED tests in detecting cancers very early, but it also sets the benchmark sensitivities required for these tests to succeed."
Moving the science from the lab to the clinic requires rigorous clinical trials to prove the reliability and safety of blood-based cancer screening tests. Regulatory approvals are also necessary before these tests can be integrated into routine medical practice. Additionally, determining the appropriate clinical follow-up after a positive test result, including further scans, biopsies, and preventive treatments, is crucial.

A Hopeful Future for Cancer Diagnostics
Despite the existing limitations, this research signifies a promising advancement in cancer diagnostics. Combined with ongoing developments in treatment, particularly therapies targeting multiple cancer types, this breakthrough holds the potential to significantly improve cancer survival rates.
Newer articles
Older articles
-
 Government issues warning for these Android smartphone and tablet users
Government issues warning for these Android smartphone and tablet users
-
 This new AI tool can help you book train tickets, get refunds and check details on IRCTC website and app
This new AI tool can help you book train tickets, get refunds and check details on IRCTC website and app
-
 Microsoft plans to take on iPhone and Android smartphones with this new device
Microsoft plans to take on iPhone and Android smartphones with this new device
-
 Mahbub Anam replaces Faruque Ahmed as new BPL chairman
Mahbub Anam replaces Faruque Ahmed as new BPL chairman
-
 India vs England: Can Bazball outplay India's new era? Key battles and what to expect
India vs England: Can Bazball outplay India's new era? Key battles and what to expect
-
 Anderson–Tendulkar Trophy: India–England Test series enters new era, tribute to cricketing legends
Anderson–Tendulkar Trophy: India–England Test series enters new era, tribute to cricketing legends
-
 Tait rues new-ball miss as Nissanka punishes Bangladesh
Tait rues new-ball miss as Nissanka punishes Bangladesh
-
 iQoo Z9 Turbo new leak reveals key specifications: All the details
iQoo Z9 Turbo new leak reveals key specifications: All the details
-
 Sachin Tendulkar bats for Pataudi legacy as new India-England trophy is unveiled
Sachin Tendulkar bats for Pataudi legacy as new India-England trophy is unveiled
-
 Teen Innovator Soars to New Heights: Mehar Singh Breaks Guinness World Record with Lightning-Fast Drone Ascent
Teen Innovator Soars to New Heights: Mehar Singh Breaks Guinness World Record with Lightning-Fast Drone Ascent